Learning objectives
ddd
ddd
ddd
Introduction
Antimicrobial susceptibility testing is a crucial process in the field of microbiology and infectious diseases. It involves determining the effectiveness of various antibiotics or antimicrobial agents against specific bacteria or other microorganisms. By conducting this testing, healthcare professionals can make informed decisions about which antibiotics or treatments will be most effective in combating infections. This testing is also performed routinely on large collections of isolates to detect epidemiological trends of resistance (surveillance).
In vitro antimicrobial testing also plays a critical role in drug discovery and early identification of
Antimicrobial spectrum
The activity spectrum of an antibacterial drug is related to the range of bacteria it can target. A narrow-spectrum antimicrobial specifically targets certain subsets of bacterial pathogens. For instance, some narrow-spectrum drugs only work against gram-positive bacteria, while others only work against gram-negative bacteria. When the pathogen causing an infection is known, it is generally best to use a narrow-spectrum antimicrobial to minimize harm to the normal microbiota.
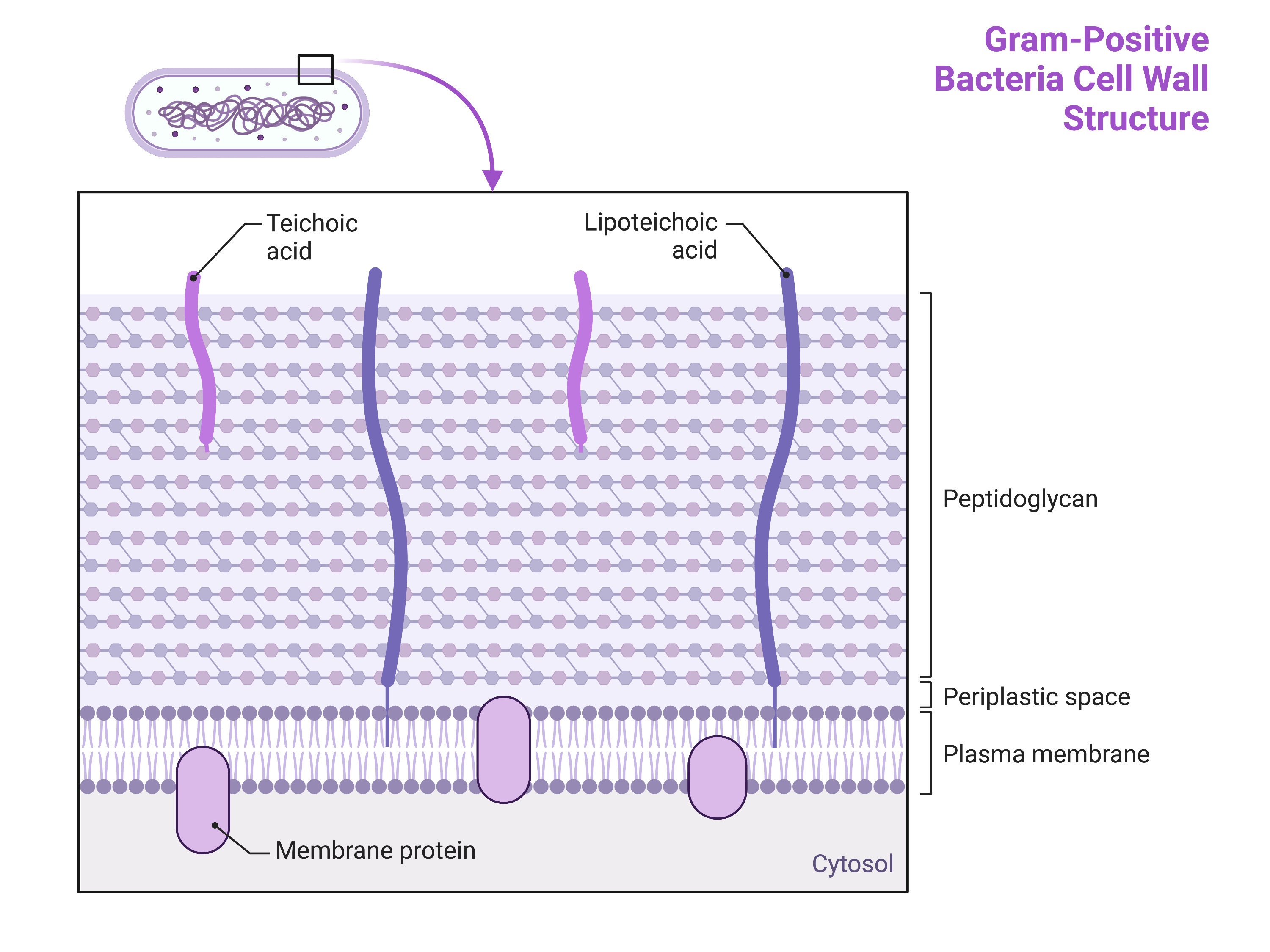
The bacterial cell wall plays a critical role in defining the spectrum of antimicrobial activity (?@fig-cellwall).
Gram-positive bacteria have a cell wall consisting of many layers of peptidoglycan totalling 30–100 nm in thickness(Figure 1). These peptidoglycan layers are commonly embedded with teichoic acids (TAs), carbohydrate chains that extend through and beyond the peptidoglycan layer. TA is thought to stabilize peptidoglycan by increasing its rigidity. TA also plays a role in the ability of pathogenic gram-positive bacteria such as Streptococcus to bind to certain proteins on the surface of host cells, enhancing their ability to cause infection.
In addition to peptidoglycan and TAs, bacteria of the family Mycobacteriaceae have an external layer of waxy mycolic acids in their cell wall. These bacteria are referred to as acid-fast, since acid-fast stains must be used to penetrate the mycolic acid layer for purposes of microscopy
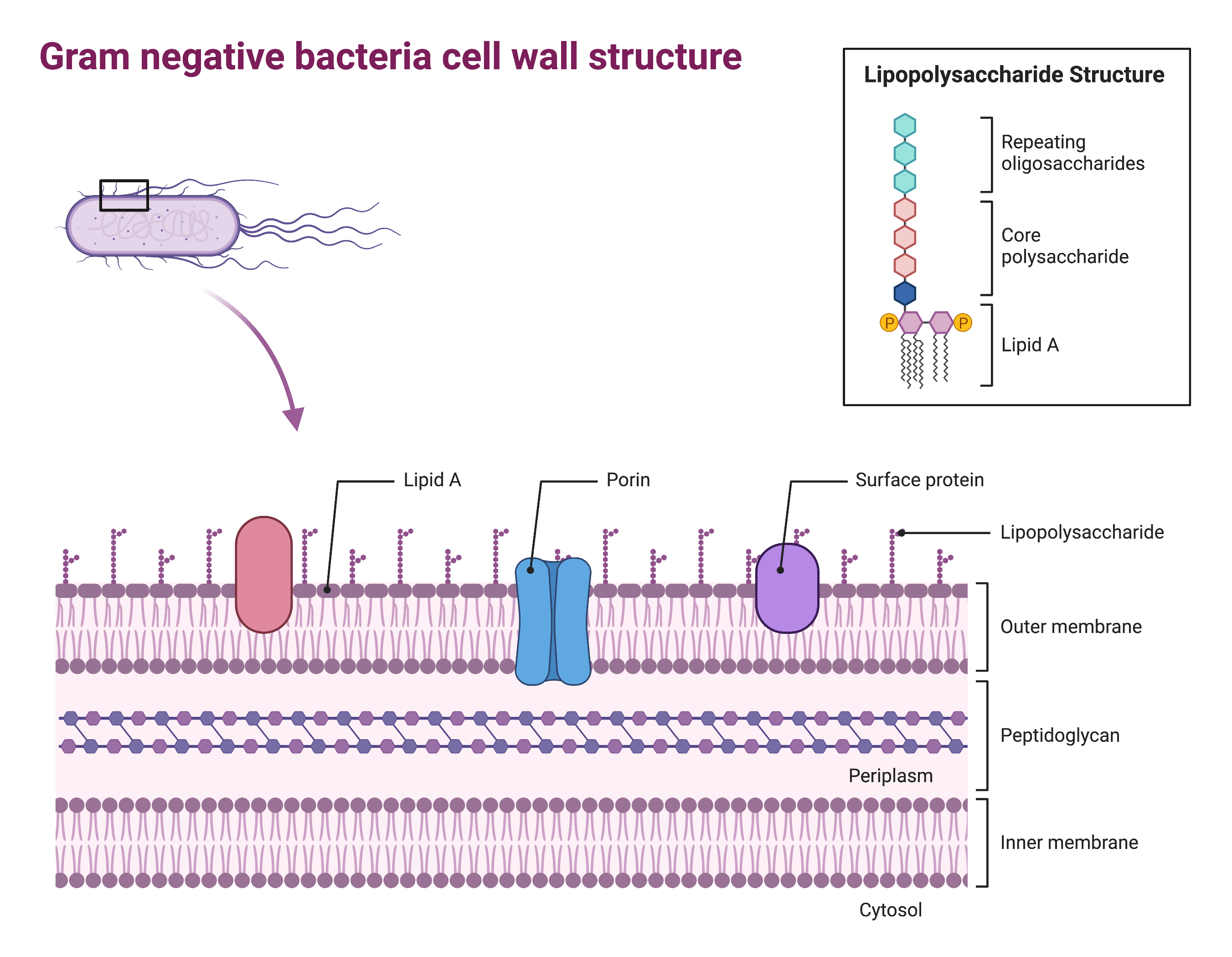
Gram-negative bacteria have a much thinner layer of peptidoglycan (no more than about 4 nm thick) than gram-positive cells, and the overall structure of their cell envelope is more complex (Figure 2). In gram-negative cells, a gel-like matrix occupies the periplasmic space between the cell wall and the plasma membrane, and there is a second lipid bilayer called the outer membrane, which is external to the peptidoglycan layer. This outer membrane is attached to the peptidoglycan by murein lipoprotein.
The outer leaflet of the outer membrane contains the molecule lipopolysaccharide (LPS), which functions as an endotoxin in infections involving gram-negative bacteria, contributing to symptoms such as fever, haemorrhaging, and septic shock.
Each LPS molecule is composed of Lipid A, a core polysaccharide, and an O side chain that is composed of sugar-like molecules that comprise the external face of the LPS (Figure 2). The composition of the O side chain varies between different species and strains of bacteria. Parts of the O side chain called antigens can be detected using serological or immunological tests to identify specific pathogenic strains like Escherichia coli O157:H7, a deadly strain of bacteria that causes bloody diarrhoea and kidney failure.
The Gram-stain test
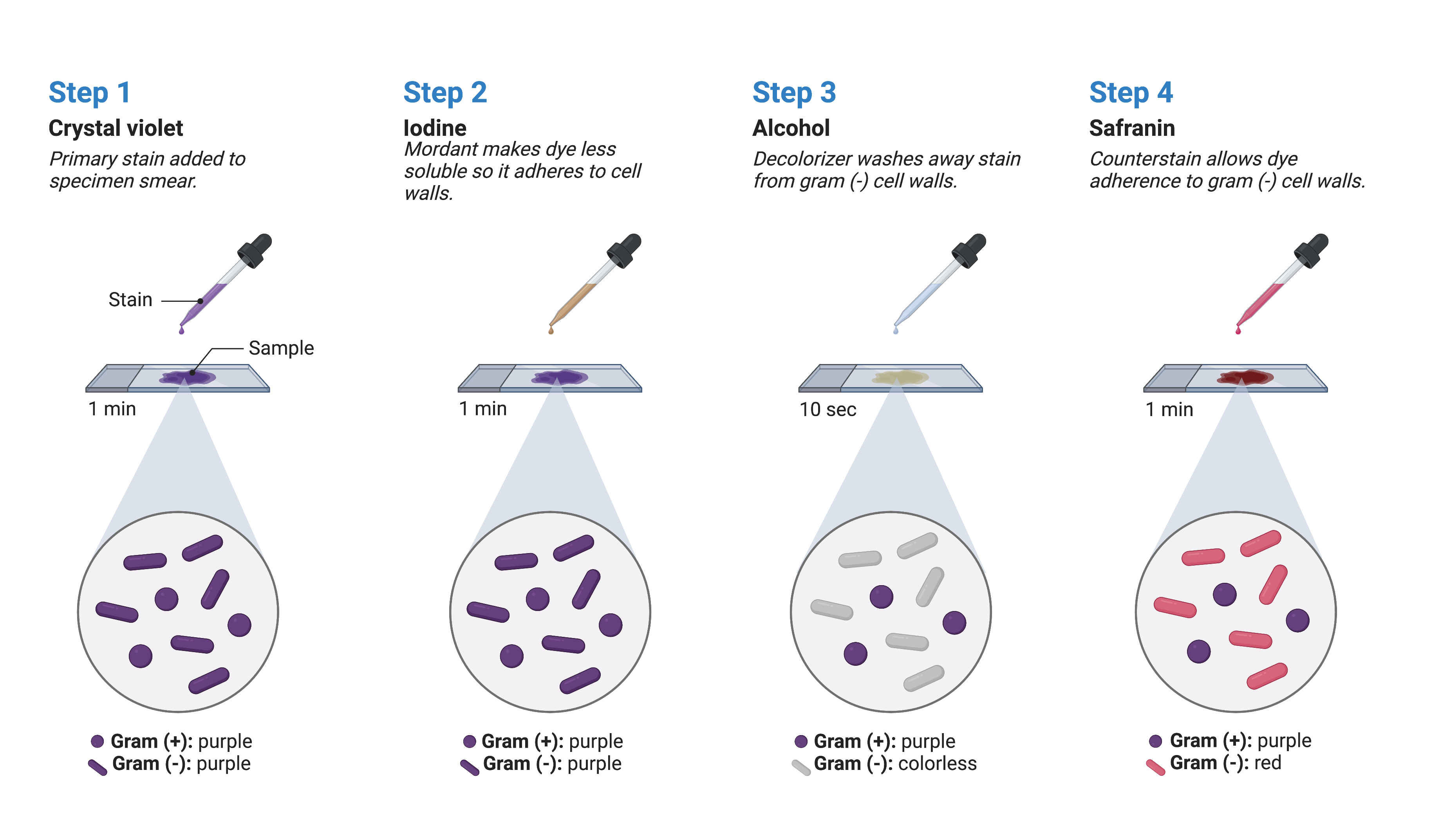
The Gram stain procedure is a widely used differential staining technique that was developed by Danish microbiologist Hans Christian Gram in 1884. It enables the differentiation of bacteria based on the characteristics of their cell walls. Even today, it remains one of the most commonly employed staining methods in microbiology and guiding antibiotic therapy. The steps involved in the Gram stain procedure are as follows (Figure 3):
The first step involves applying crystal violet, a primary stain, to a heat-fixed smear. This primary stain imparts a purple colour to all the cells on the slide.
Gram’s iodine, an essential component called a mordant, is added as the second step. A mordant acts as a fixing agent, and in this context, Gram’s iodine acts as a trapping agent that forms complexes with the crystal violet. These complexes accumulate in the thick peptidoglycan layers of the cell walls.
The third step involves adding a decolourising agent, which is typically ethanol or an acetone/ethanol solution. The decolourising agent has a different effect on cells with varying thicknesses of peptidoglycan layers. Those cells with thick peptidoglycan layers are less affected by the decolourising agent, allowing them to retain the purple crystal violet dye and appear purple. In contrast, cells with thinner peptidoglycan layers are more easily decoulorised and lose the crystal violet stain, appearing colourless.
Finally, a secondary counterstain, usually safranin, is applied. Safranin stains the decolourised cells pink, allowing them to be more easily visualized. However, cells that still contain the retained crystal violet dye are less noticeable since the purple colour masks the pink stain of the counterstain.
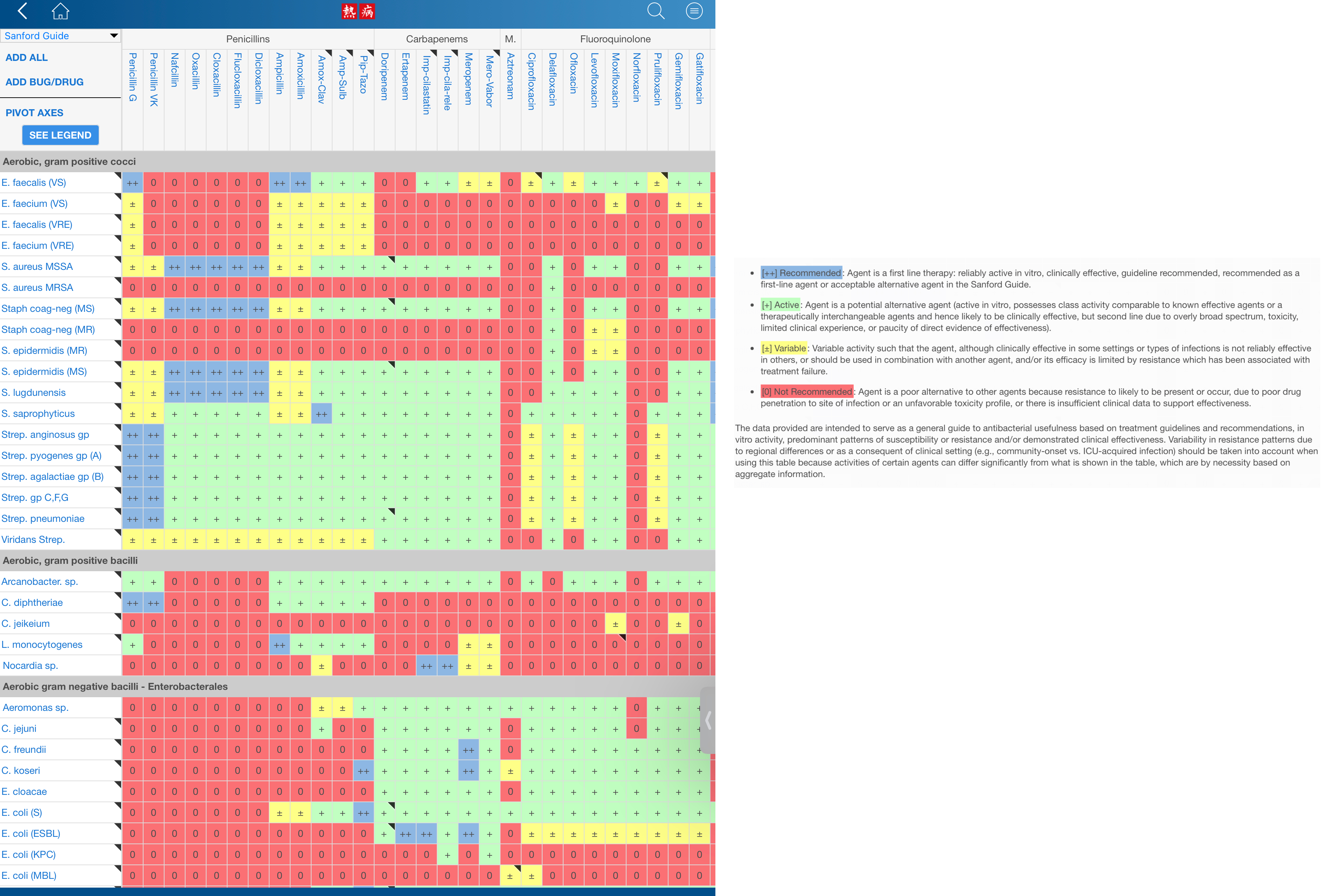
Clinicians use Gram stain results prior to definitive pathogen identification along with knowledge of the most likely pathogens associated with the clinical presentation/differential diagnosis of infection in the patient to select antimicrobial therapy. Spectrum of activity tables such as those compiled in the Sanford Guide and accessible in cell phone applications can be consulted to select an antimicrobial therapy with appropriate spectrum of activity (Figure 4).
Why not prescribe the broadest-spectrum antimicrobial for all infections?
Some broad-spectrum antibiotics can target a wide variety of bacterial pathogens, including both gram-positive and gram-negative species. Broad-spectrum antibiotic therapy may be used as empiric therapy while waiting for laboratory identification of the infecting pathogen.
Broad-spectrum antimicrobials are frequently used for polymicrobic infections (infections with multiple bacterial species), infectious in immunocompromised patients (where the spectrum of potential pathogens is larger), and for prophylactic prevention of infections for certain surgies or invasive procedures. In some cases, a broad-spectrum antimicrobial may be chosen to treat an infection when a narrow-spectrum drug fails due to the development of drug resistance by the target pathogen.
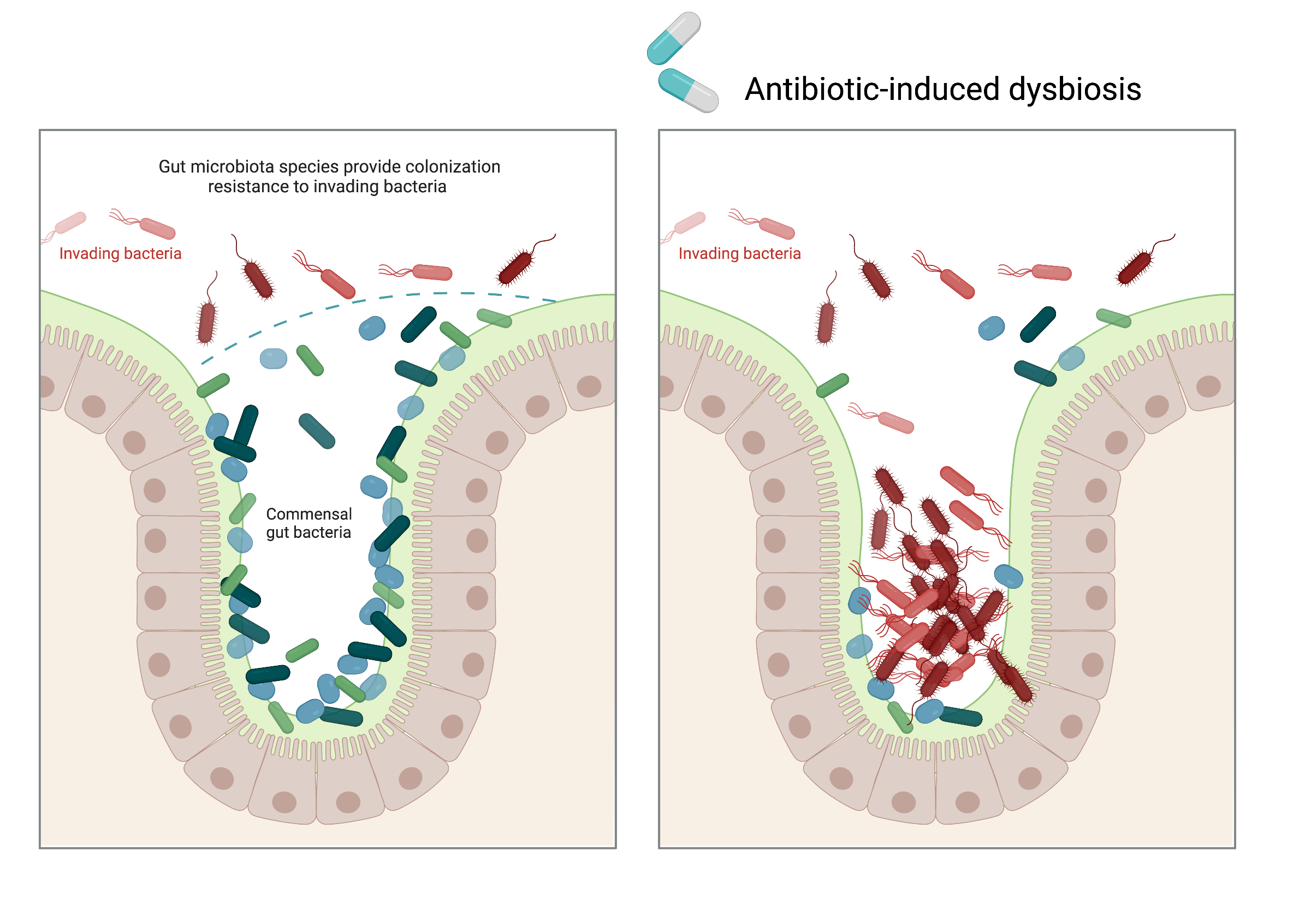
However, using broad-spectrum antimicrobials comes with the risk of also targeting a wide range of normal microbiota (Figure 5). This increases the risk of a superinfection, which is a secondary infection that occurs in a patient who already has a pre-existing infection.
Superinfections can happen when the antibacterial intended for the original infection kills off the protective microbiota, allowing another pathogen that is resistant to the antibacterial to multiply and cause a secondary infection. Examples of superinfections that can develop due to antimicrobial usage include yeast infections (candidiasis), multidrug resistant gram-positive and gram-negative pathogens, and pseudomembranous colitis caused by Clostridium difficile, which can be life-threatening.
Bactericidal versus bacteriostatic activity
Antibacterial drugs can have different effects on target bacteria. Some drugs are bacteriostatic, meaning they temporarily inhibit bacterial growth, but the bacteria can resume growth once the drug is eliminated. On the other hand, bactericidal drugs actually kill the target bacteria.
The choice between using bacteriostatic or bactericidal drugs depends on factors such as the type of infection and the patient’s immune status. In patients with strong immune defences, both types of drugs can be effective in achieving a clinical cure. However, for immunocompromised patients, using a bactericidal drug is crucial for successfully treating infections.
In the case of life-threatening infections like acute endocarditis or meningitis, bactericidal antibiotics are generally favoured. However, in recent years the dogma of “bactericidal activity” has been questioned by some infectious diseases specialists as limited data support the superiority of outcomes for regimens defined as bactericidal versus bacteriostatic in them microbiology laboratory.
Is the commonly held belief that bactericidal activity is crucial for certain infections actually challenged by clinical outcome data, as suggested by this analysis conducted by Wald-Dickler and colleagues?